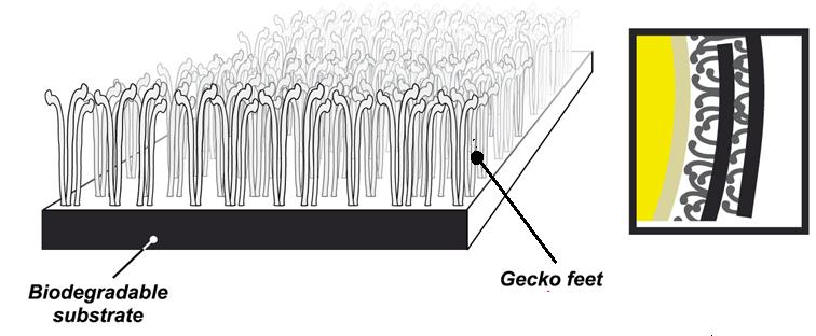
BioJoinTM Sutureless Connectors* These biodegradable tissue-connectors combine the use of novel proprietary polymeric thermosensitive bioadhesives (such as polyNIPAM, which has the reversible property of being a liquid at room temperature and an adhesive at body temperature) and surface designs (emulating gecko-feet as illustrated in the bottom figure) for a quick, easy and strong snap-on sutureless anastomosis of soft-tissue structures, such as blood vessels, nerves, trachea, intestines, urethra etc. This eliminates neurorrhaphy from neurosurgery: the time consuming, expensive and often cumbersome process of suturing at hard-to-access sites at sub-millimeter diameters, requiring sophisticated equipment, highly-skilled surgeons and specialized facilities. Similarly, application-optimized designs can cater to a wide gamut of surgeries, such as radical prostectomy, aneurysm repairs, facial neurorrhaphy, tracheal and intestinal anastomoses, and even cardiac bypass. Extensive surveys indicate that such a solution can save up to 40% surgical time, provide immense clinical benefits, broadly open up PNS neurosurgeries and other hitherto-exclusive microsurgical procedures even onto field clinics, and also generate great cost savings for healthcare providers. In the case of neurosurgeries, the clinical benefits include reduced risk of neuroma formation, thromboembolism, granulomatous inflammation, surgical site infections and complications from anesthesia; as well as faster and better functional recovery. The following movie illustrates the proposed combined use of Innerva and BioJoin for repairing a peripheral nerve injury:
|
InnervaTM
Nanofiber Nerveguides* These next-generation biodegradable nerve guides, are the first-ever "active" guides: shown to be able to greatly accelerate the axon regeneration rate across a trauma-severed hiatus by up to seven times as the current passive hollow tubes, in our rodent studies. This could remove the biggest bottleneck for regenerative alternatives to autologous sural grafts: the current length limitation of only 2cm. Such a 100% regenerative solution, possibly viable even for brachial plexus trauma, can provide a much improved, safer and quicker functional recuperation, avoiding the surgery and loss-of-function at a donor-site. Electrospun nanofibers encased in Innerva form a trellis that stimulates directed migration of Schwann cells from the proximal to distal ends of a lacuna. Axon formation and vascular in-growth can also be further enhanced through a chemotactic gradient of GDNF-based neurotrophic growth factors. planned for a second generation. The top figure shows the proposed use of a Innerva nerve guide to treat a nerve injury: please note the longitudinally aligned PCLEEP nanofibers in the inset. The second figure shows the comparison between regenerated rat sciatic nerves using a commercially available collagen-based hollow nerve guide from a competitor and a Innerva nerveguide, after the same timeline. Please note the epineurium is poorly filled in the former, and the electromyographic evidence shows a far superior recovery and axon density in the latter. The bottom figure contrasts the axon regeneration densities between (a) Hollow Collagen tube (current standard), (b) Innerva 1st Gen, and (c) Innerva 2nd Gen that also uses neurotrophic factors. 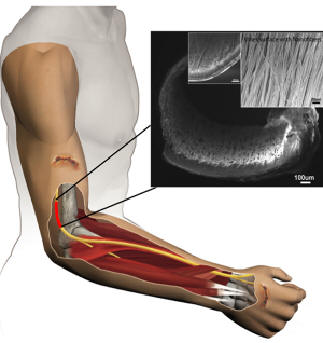   |
Presentations
Demo, Dr. Kylstra >> Concept Movies** Innerva >> BioJoin >> Together >> |
By
completely avoiding sural nerve graft harvesting and microsuturing
of nerves and vascular endings at miniature diameters, these
quantum-leap technologies hold the promise to be practice-altering
in the way neurovascular surgeries are done today, and to greatly
reduce their duration, risks, cost and complexity.
* PS: Please note that the technologies described above are under consideration of the FDA, and their description here is only meant to be informative on our research findings on animals. Ratner BioMedical Inc., or any of its officers or affiliated parties, do not make any representations or suggestions, either expressed or implied, as to the safety or efficacy of using these technologies in humans, which we are not in a position to make until the appropriate FDA clearances are received.
**Requires free VLC Player or Apple QuickTime.